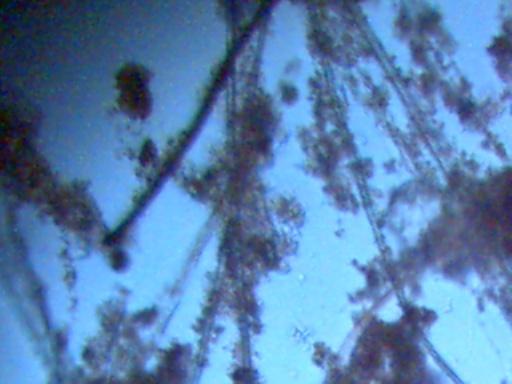
RAINWATER SAMPLES:
MICROSCOPE VIEWS (II)
Clifford E Carnicom
Aug 16 2001
Santa Fe NM
The following photographs of rainwater concentrate as viewed under the microscope are offered with limited interpretation. This page will serve primarily as a log of recurring structures which are found under various conditions. As further information is acquired regarding the identity of certain materials, it will be provided. All citizens are urged to participate in the process of further collection of rainfall samples, subsequent distillation or concentration and the identification of material substances within. Any assistance provided by other researchers or sources is welcome. The majority of the photographs are taken at a magnification of approximately 500x.
If sufficient rainfall is available, the water is now commonly being reduced by approximately 99% in volume. In two cases, approximately 400ml (~2cups) of rainfall was reduced to a volume of approximately 4ml.
Users may also refer to the initial investigation on this page. Crystal examinations as described on previous pages may also be of interest to readers. Additional microscopic images are available as well.
Additional short descriptions and captions for these microphotographs without comments will be provided in the near future.

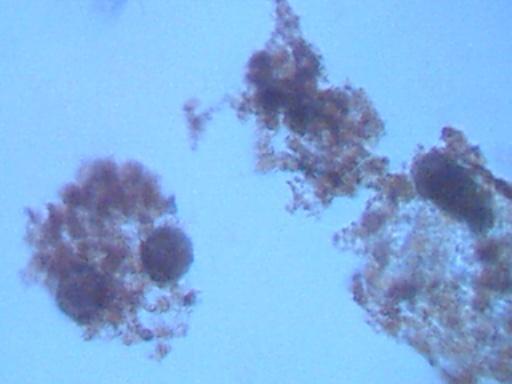
Magnification approx. 500x.
Fibrous materials occurring frequently within samples.
A dominant material of the samples appears to be an oxide form,
which appears to be attracted to fibrous elements when they are present.
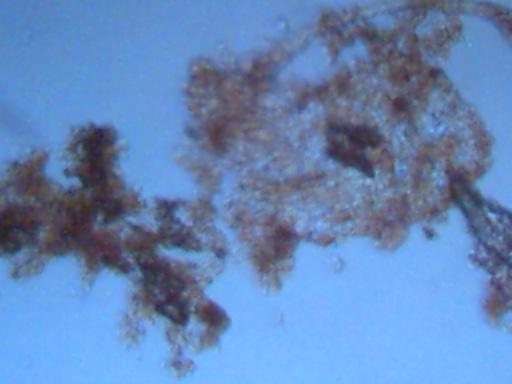
Magnification approx. 500x.
This is one of the visually dominant materials found within the rainfall
samples that are subjected to the heat of boiling or distillation.
At this stage of investigation, it appears to be an metallic oxide form.
Further assistance of identification through chemical analysis is invited.

Magnification Approx. 2000x
The appearance of the spherical structures shown has been difficult to
detect. Although these forms have been visible in an unaltered rainfall
concentrate sample, they have been brought to greater prominence and visibility
by the addition of a small amount of sulfuric acid (approx. 2 drops per 4ml).
The acid appears to dissolve the apparent oxide form which is
dominant in both size and visibility to most samples, but does not
appear to affect the spherical components. The spherical structures
shown are essentially transparent and difficult to both see and photograph.
In a reference book on aerosols that has been consulted, it is of interest to
note that aluminum particulates are shown within a photograph as being
spherical in shape. The materials shown measure at approximately 2 microns
for each sphere (human hair approx. 60-100microns thick).

Magnification Approx. 2000x
Another example of the spherical components which have been described above,
readily visible and isolated after the introduction of a couple of drops of
sulfuric acid into the rainfall concentrate sample. It is also of interest that
the remaining sample within the test tube that has been treated in this manner
visibly shows what appears to abundant metallic particulate matter within it.
Although no claim at this time will be made that this material is aluminum,
it does satisfy the expected visible properties of that element. When the test
tube is agitated, the highly reflective particulate matter can be seen to adhere
and gradually descend on the inside of the glass wall of the test tube.
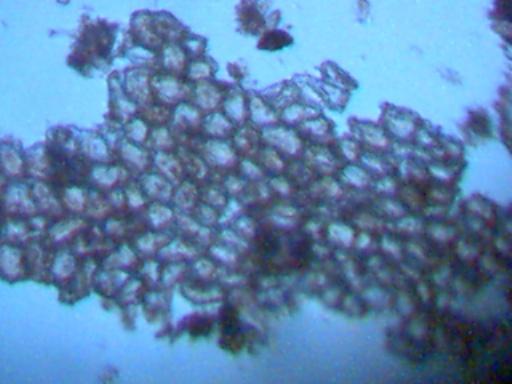
Magnification approx. 500x.
This material is being shown because of its repeated presence.
It has been dismissed on several occasions because it has been assumed
to be of a spurious plant origin. The repeated appearance of this cellular structure
establishes the need for positive identification of it.

Magnification approx. 500x.
Another example of the cellular layer material that is repeating within different samples
that have been viewed under the microscope. Strong consideration must be given to the
possibility of a plant origin or contaminant with this material. It is reoccurring, however,
and it maintains this form after subjection to heat. It is presented because of the
need for identification that exists.
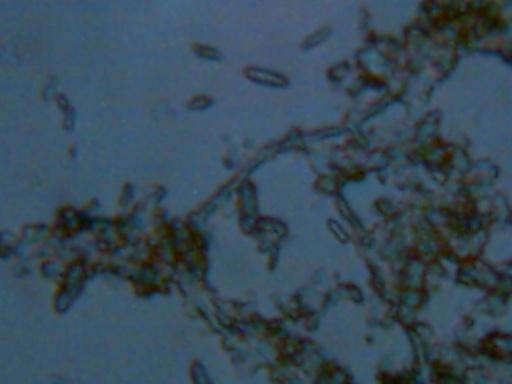
Magnification approx. 2000x.
This photograph shows two primary components. The first is a fibrous component,
which forms the backdrop of the image. In addition, numerous rod shaped objects
appear within this image. These rods are quite small and numerous within the
sample shown. This is an image of what remains after a portion of a wet slide
mount has dried. The higher magnification increases the difficulty of light
collection under the microscope. The objects have been measured at
approximately 1-2 microns in thickness, and approximately 5 microns in
length. A human hair is approximately 60-100 microns thick.
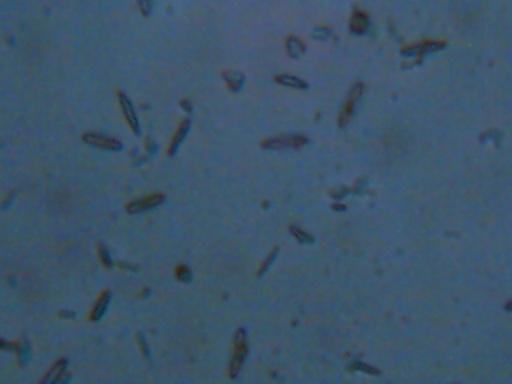
Magnification Approx. 2000x
Another image of the rod-shaped features that are visible under one sample
of a wet slide mount that has been allowed to dry.

Magnification Approx. 500x
This is a distinctive crystal that is forming along the perimeter of a
rainfall concentrate sample that has been treated with a small amount
of sulfuric acid (approx. 2 drops per 4ml of rainfall concentrate).

Magnification Approx. 500x
Another example of a distinctive crystal that forms under the conditions
which have been described immediately above.

Magnification Approx. 500x
This photograph is dominated with by what appears to be a metal oxide form
as has been described earlier. One strong candidate for testing will be magnesium
oxide, due to earlier test results with unheated rain water samples. This sample
also has the presence of larger circular or spherical objects. At this point
these are not to be considered recurring components. Because of their size,
strong consideration should be given to the possibility of being a pollen grain.
In the past, however, both pine and juniper pollen grains have been identified,
(both of which are expected in this southwestern region), and these are not
similiar to form of either of these pollen types. It must also be remembered
that these rain sample concentrates have been subject to the heat of distillation
or evaporation by boiling. If the structures shown continue to appear under
further observation, they will also require positive identification.