RAINWATER SAMPLES: MICROSCOPE VIEWS (II)
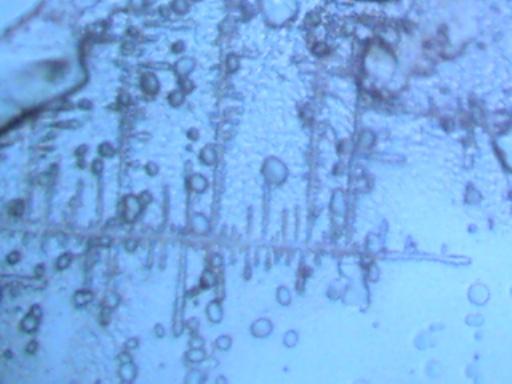
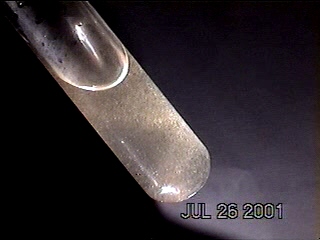
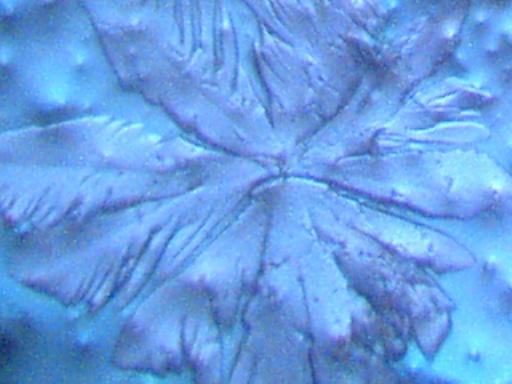
More photographs of rainwater concentrate as viewed under a microscope are presented here as a complement to investigations recently presented on the Carnicom.com website (see previous RAINWATER METALS, CRYSTAL CHEMISTRY, and RAINWATER SAMPLES: MICROSCOPE VIEWS papers). These photographs depict primarily a log of recurring structures which are found under various conditions, rather than an analysis of such structures. These structures in these microscope pictures appear to be fibers, metal oxides, and other unidentified materials.
All citizens are urged to participate in the process of further collection of rainfall samples, subsequent distillation or concentration and the identification of material substances within. Any assistance provided by other researchers or sources is welcome.