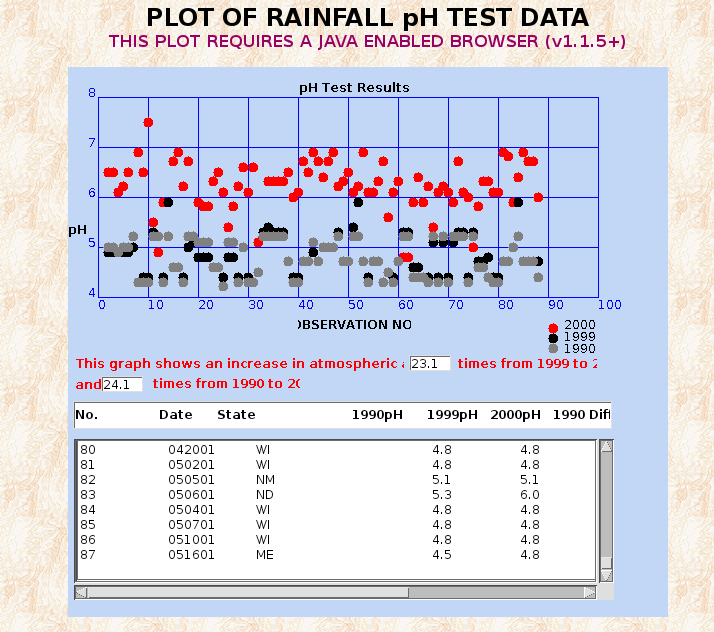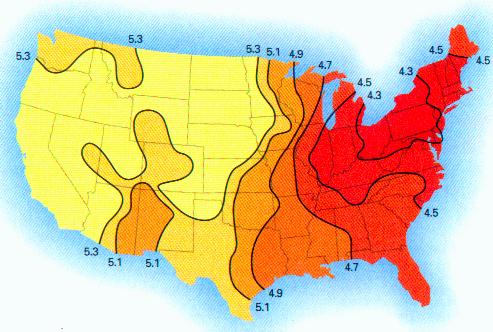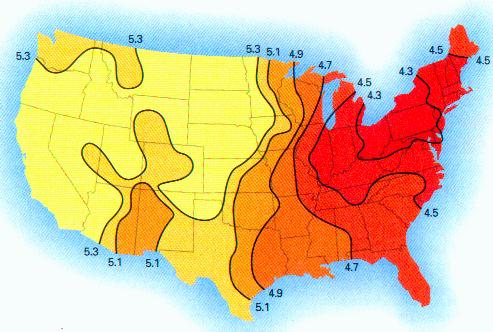
MORGELLONS : pH, CONDUCTIVITY, IONS & LIVE ANALYSIS
All work thus far indicates that the culture forms under examination encompass primary pathogenic forms that are in association with the so-called "Morgellons" condition. These are the the encasing filament, the chlamydia-like organism, the mycoplasma-like (pleomorphic) organism and under certain conditions, the erythrocytic (red blood cell) form. This list does not exclude current or future discoveries by any party that are sufficiently documented, but this list is inclusive as of this date.